How soap works
The most common and best known function of soap is to help in removal of dirt which cannot be removed by water alone. Grease, oils and other organic compounds tend to stick to the cleaning surface rather than to be washed away, while mineral dirt tends to stick to grease. To remove all of it, greasy dirt must be treated with special chemicals which enable its mixing with water. Such chemicals are called surfactants, and soaps, shampoos and laundry detergents are best known members of this group of chemicals.
Surfactants
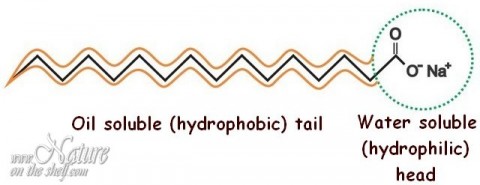
Surfactants (this term is derived from “surface active agents”) are compounds that reduce surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid. Lower surface tension enables better spreadability of liquid and its better wetting properties. Amongst other actions, surfactants act as cleansers, foaming agents, and emulsifiers. A common feature of all types of surfactants is the dual character of their molecules. Each surfactant molecule has both a polar or water-soluble (hydrophilic) and a non-polar or oil-soluble (lipophilic, hydrophobic) part. Due to this feature, such chemicals are also called amphiphilic or amphipathic. The polar (water-soluble) part of the surfactant molecule can be negatively or positively charged, or it can contain both positive and negative charges, or may not be charged at all. Depending on this feature, surfactants are divided into ionic (anionic or cationic), amphoteric, and non-ionic.
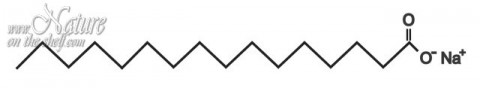
Soap is an anionic surfactant. The water-soluble part of the soap molecule (carboxylate part, also called a “head”) is the molecule end where sodium is attached. When soap is dissolved in water, molecules of water interact with sodium, and the head of soap molecule becomes negatively charged (anionic). The oil-soluble (hydrophobic, water-repelling) part of the soap molecule is a non-polar “tail” which originates from hydrocarbon chain of fatty acid.

Soap micelles
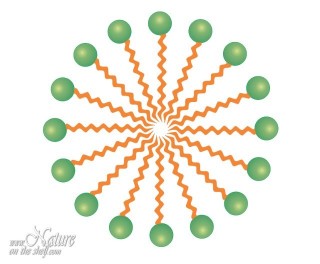
When soap molecules are dissolved in water, the water-repelling hydrophobic tails cluster together while hydrophilic heads surround them arranging themselves in a spherical form toward water molecules. Such spheres, called micelles, are dispersed throughout the water. This enables soap to act as an emulsifier i.e. enables mixing water and oils. Hydrocarbon chains (non-polar ”tails”) of soap molecules are attracted by non-polar substances like grease and other organic matter. They surround these substances and capture them inside of the soap micelles, while hydrophilic “heads” are attracted by water molecules and allow the micelles to float in water. Since micelle surfaces are negatively charged, they repel each other preventing coalescence.
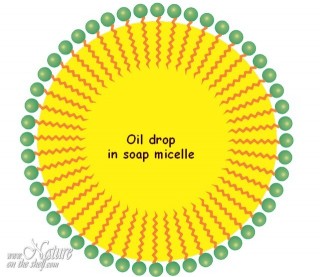
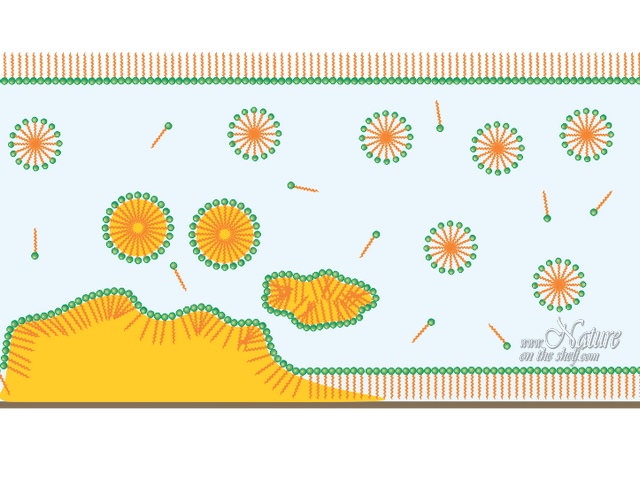
An emulsifying action of soap is enforced by friction (e.g. rubbing hands or whirling the clothes in the washing machine) that breaks grease into small droplets, mixing them with soap micelles and allowing easier emulsification. In this way, soap creates an emulsion of grease in water which can be easily rinsed away. Since mineral dirt sticks to grease, it will be removed simultaneously with the grease.
Disadvantages of soap
Soap is an excellent cleanser, but in certain circumstances soap has some disadvantages. When soap is used in hard water, ions of calcium and magnesium from water react with water-soluble sodium soap molecules and replace sodium, forming water-insoluble salts of fatty acids. These salts form a waxy solid, known as soap scum that is often seen on sinks and bathtubs. This is the reason why hard water needs larger amounts of soap in comparison with soft water in order to achieve the same lathering and cleaning effects.
When soap is used for washing clothes in hard water, soap scum forms deposits on the fabric and, with time, causes discoloration of clothes from white to yellow or gray. If soap is used as hair wash in hard water, soap scum will remain attached on hair and make the hair dull and hard to comb.
If soap is used in acidic water, acid converts soap (fatty acid salts) into free fatty acids. Free fatty acids are less soluble than salt and precipitate forming scum, thus making soap unusable in acidic water.
